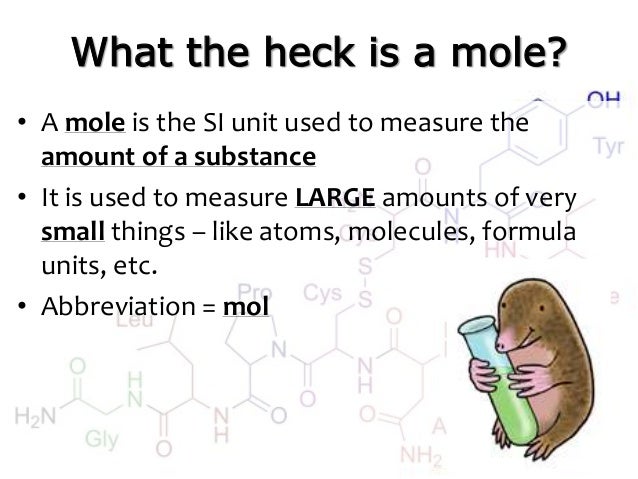
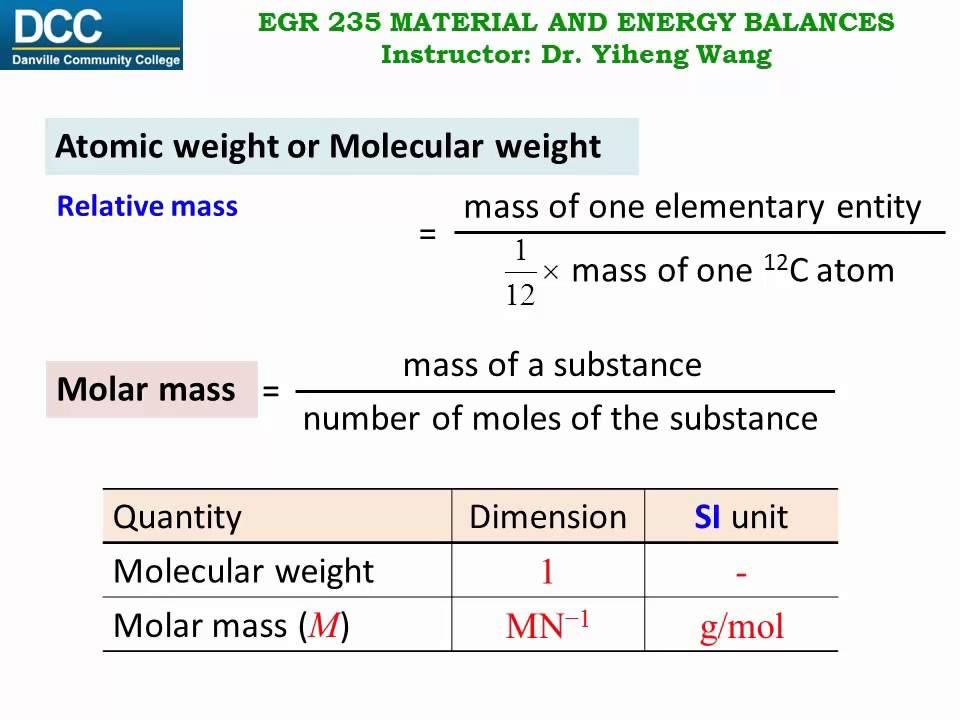
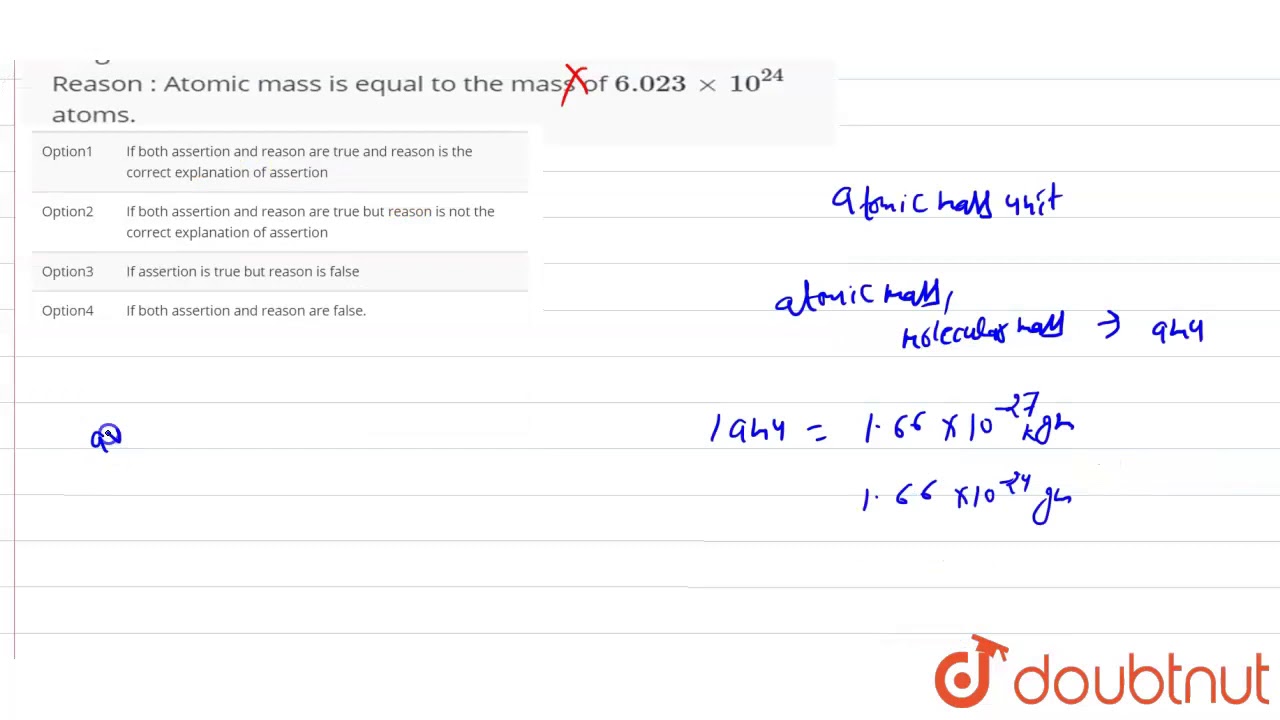
Moles Notes. 1. Atomic Mass Unit amu – atomic mass unit, used to describe the mass of an atom Conversion factor: 1 amu = 1.66 x g Equivalence statement: - ppt download
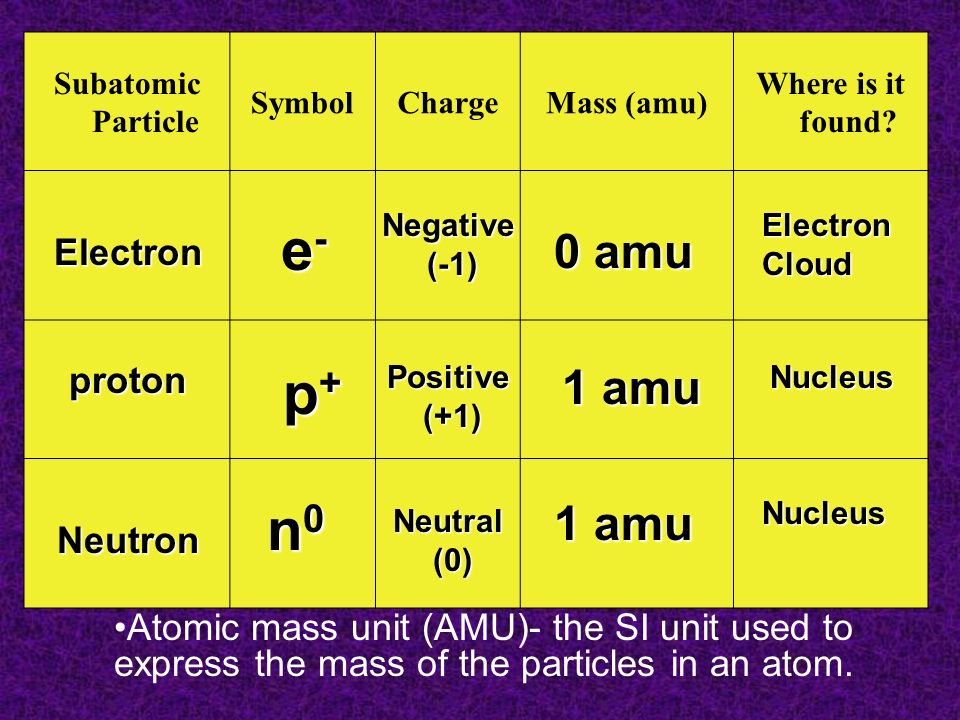
Subatomic Particle SymbolChargeMass (amu) Where is it found? Atomic mass unit (AMU)- the SI unit used to express the mass of the particles in an atom. - ppt download

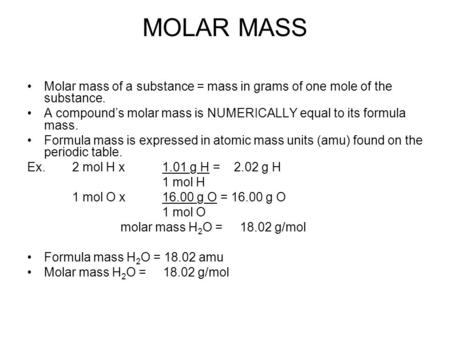
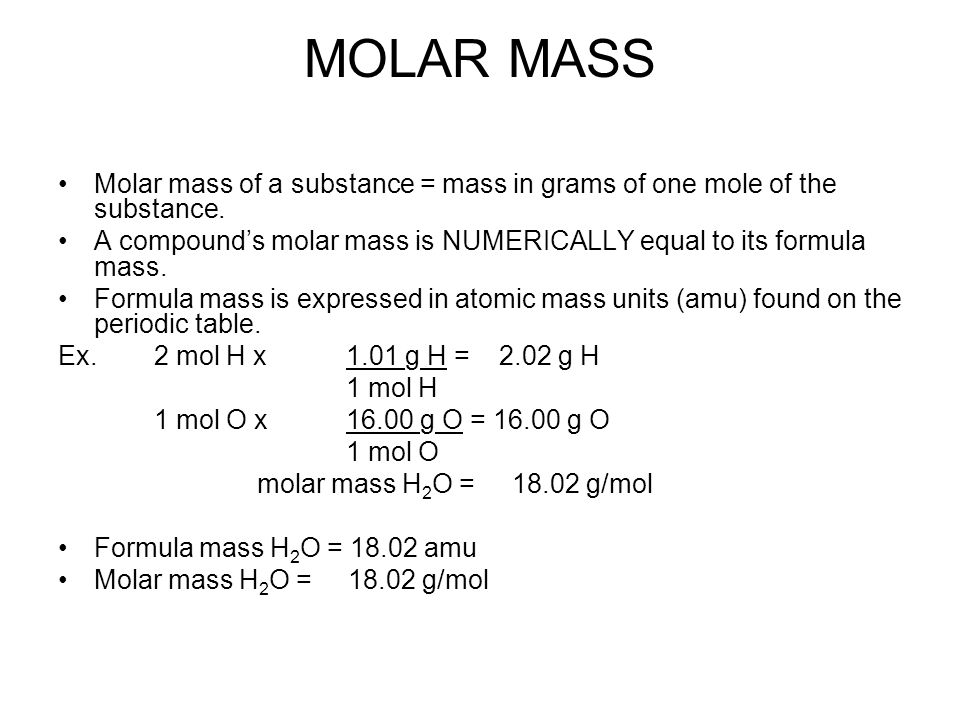
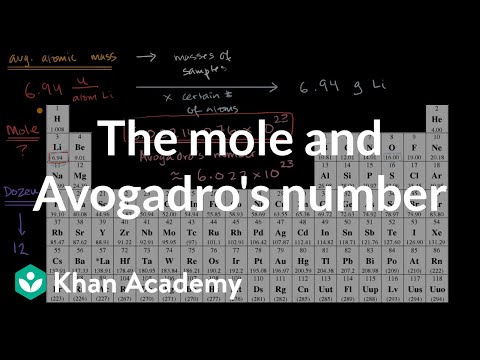
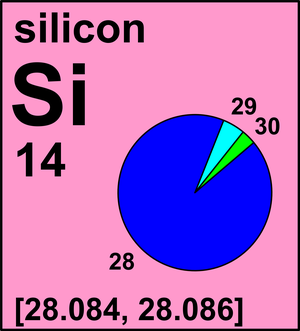
The Mole 1 Molecular and Formula Mass 2 What is the average atomic mass of the following atoms? What unit is used? Oxygen 16.00amu Zinc 65.38amu Silver. - ppt download







/hands-cupping-a-glowing-atom-in-the-studio-164210758-5b259c6b04d1cf0036d0b90f.jpg)